Brings Seurat to the tidyverse!
website: stemangiola.github.io/tidyseurat/
Please also have a look at
- tidyseurat for tidy single-cell RNA sequencing analysis
- tidySummarizedExperiment for tidy bulk RNA sequencing analysis
- tidybulk for tidy bulk RNA-seq analysis
- tidygate for adding custom gate information to your tibble
- tidyHeatmap for heatmaps produced with tidy principles

Introduction
tidyseurat provides a bridge between the Seurat single-cell package [@butler2018integrating; @stuart2019comprehensive] and the tidyverse [@wickham2019welcome]. It creates an invisible layer that enables viewing the Seurat object as a tidyverse tibble, and provides Seurat-compatible dplyr, tidyr, ggplot and plotly functions.
Functions/utilities available
| Seurat-compatible Functions | Description |
|---|---|
all |
| tidyverse Packages | Description |
|---|---|
dplyr |
All dplyr APIs like for any tibble |
tidyr |
All tidyr APIs like for any tibble |
ggplot2 |
ggplot like for any tibble |
plotly |
plot_ly like for any tibble |
| Utilities | Description |
|---|---|
tidy |
Add tidyseurat invisible layer over a Seurat object |
as_tibble |
Convert cell-wise information to a tbl_df
|
join_features |
Add feature-wise information, returns a tbl_df
|
aggregate_cells |
Aggregate cell gene-transcription abundance as pseudobulk tissue |
Installation
From CRAN
install.packages("tidyseurat")From Github (development)
devtools::install_github("stemangiola/tidyseurat")Create tidyseurat, the best of both worlds!
This is a seurat object but it is evaluated as tibble. So it is fully compatible both with Seurat and tidyverse APIs.
pbmc_small = SeuratObject::pbmc_smallIt looks like a tibble
pbmc_small##
[90m# A Seurat-tibble abstraction: 80 × 15
[39m
##
[90m#
[90mFeatures=230 | Cells=80 | Active assay=RNA | Assays=RNA
[0m
[39m
## .cell orig.ident nCount_RNA nFeature_RNA RNA_snn_res.0.8 letter.idents groups
##
[3m
[90m<chr>
[39m
[23m
[3m
[90m<fct>
[39m
[23m
[3m
[90m<dbl>
[39m
[23m
[3m
[90m<int>
[39m
[23m
[3m
[90m<fct>
[39m
[23m
[3m
[90m<fct>
[39m
[23m
[3m
[90m<chr>
[39m
[23m
##
[90m 1
[39m ATGC… SeuratPro… 70 47 0 A g2
##
[90m 2
[39m CATG… SeuratPro… 85 52 0 A g1
##
[90m 3
[39m GAAC… SeuratPro… 87 50 1 B g2
##
[90m 4
[39m TGAC… SeuratPro… 127 56 0 A g2
##
[90m 5
[39m AGTC… SeuratPro… 173 53 0 A g2
##
[90m 6
[39m TCTG… SeuratPro… 70 48 0 A g1
##
[90m 7
[39m TGGT… SeuratPro… 64 36 0 A g1
##
[90m 8
[39m GCAG… SeuratPro… 72 45 0 A g1
##
[90m 9
[39m GATA… SeuratPro… 52 36 0 A g1
##
[90m10
[39m AATG… SeuratPro… 100 41 0 A g1
##
[90m# ℹ 70 more rows
[39m
##
[90m# ℹ 8 more variables: RNA_snn_res.1 <fct>, PC_1 <dbl>, PC_2 <dbl>, PC_3 <dbl>,
[39m
##
[90m# PC_4 <dbl>, PC_5 <dbl>, tSNE_1 <dbl>, tSNE_2 <dbl>
[39mBut it is a Seurat object after all
pbmc_small@assaysPreliminary plots
Set colours and theme for plots.
# Use colourblind-friendly colours
friendly_cols <- c("#88CCEE", "#CC6677", "#DDCC77", "#117733", "#332288", "#AA4499", "#44AA99", "#999933", "#882255", "#661100", "#6699CC")
# Set theme
my_theme <-
list(
scale_fill_manual(values = friendly_cols),
scale_color_manual(values = friendly_cols),
theme_bw() +
theme(
panel.border = element_blank(),
axis.line = element_line(),
panel.grid.major = element_line(size = 0.2),
panel.grid.minor = element_line(size = 0.1),
text = element_text(size = 12),
legend.position = "bottom",
aspect.ratio = 1,
strip.background = element_blank(),
axis.title.x = element_text(margin = margin(t = 10, r = 10, b = 10, l = 10)),
axis.title.y = element_text(margin = margin(t = 10, r = 10, b = 10, l = 10))
)
)We can treat pbmc_small effectively as a normal tibble for plotting.
Here we plot number of features per cell.
pbmc_small %>%
ggplot(aes(nFeature_RNA, fill = groups)) +
geom_histogram() +
my_theme
Here we plot total features per cell.
pbmc_small %>%
ggplot(aes(groups, nCount_RNA, fill = groups)) +
geom_boxplot(outlier.shape = NA) +
geom_jitter(width = 0.1) +
my_theme
Here we plot abundance of two features for each group.
pbmc_small %>%
join_features(features = c("HLA-DRA", "LYZ"), shape = "long") %>%
ggplot(aes(groups, .abundance_RNA + 1, fill = groups)) +
geom_boxplot(outlier.shape = NA) +
geom_jitter(aes(size = nCount_RNA), alpha = 0.5, width = 0.2) +
scale_y_log10() +
my_theme
Preprocess the dataset
Also you can treat the object as Seurat object and proceed with data processing.
pbmc_small_pca <-
pbmc_small %>%
SCTransform(verbose = FALSE) %>%
FindVariableFeatures(verbose = FALSE) %>%
RunPCA(verbose = FALSE)
pbmc_small_pca##
[90m# A Seurat-tibble abstraction: 80 × 17
[39m
##
[90m#
[90mFeatures=220 | Cells=80 | Active assay=SCT | Assays=RNA, SCT
[0m
[39m
## .cell orig.ident nCount_RNA nFeature_RNA RNA_snn_res.0.8 letter.idents groups
##
[3m
[90m<chr>
[39m
[23m
[3m
[90m<fct>
[39m
[23m
[3m
[90m<dbl>
[39m
[23m
[3m
[90m<int>
[39m
[23m
[3m
[90m<fct>
[39m
[23m
[3m
[90m<fct>
[39m
[23m
[3m
[90m<chr>
[39m
[23m
##
[90m 1
[39m ATGC… SeuratPro… 70 47 0 A g2
##
[90m 2
[39m CATG… SeuratPro… 85 52 0 A g1
##
[90m 3
[39m GAAC… SeuratPro… 87 50 1 B g2
##
[90m 4
[39m TGAC… SeuratPro… 127 56 0 A g2
##
[90m 5
[39m AGTC… SeuratPro… 173 53 0 A g2
##
[90m 6
[39m TCTG… SeuratPro… 70 48 0 A g1
##
[90m 7
[39m TGGT… SeuratPro… 64 36 0 A g1
##
[90m 8
[39m GCAG… SeuratPro… 72 45 0 A g1
##
[90m 9
[39m GATA… SeuratPro… 52 36 0 A g1
##
[90m10
[39m AATG… SeuratPro… 100 41 0 A g1
##
[90m# ℹ 70 more rows
[39m
##
[90m# ℹ 10 more variables: RNA_snn_res.1 <fct>, nCount_SCT <dbl>,
[39m
##
[90m# nFeature_SCT <int>, PC_1 <dbl>, PC_2 <dbl>, PC_3 <dbl>, PC_4 <dbl>,
[39m
##
[90m# PC_5 <dbl>, tSNE_1 <dbl>, tSNE_2 <dbl>
[39mIf a tool is not included in the tidyseurat collection, we can use as_tibble to permanently convert tidyseurat into tibble.
pbmc_small_pca %>%
as_tibble() %>%
select(contains("PC"), everything()) %>%
GGally::ggpairs(columns = 1:5, ggplot2::aes(colour = groups)) +
my_theme
Identify clusters
We proceed with cluster identification with Seurat.
pbmc_small_cluster <-
pbmc_small_pca %>%
FindNeighbors(verbose = FALSE) %>%
FindClusters(method = "igraph", verbose = FALSE)
pbmc_small_cluster##
[90m# A Seurat-tibble abstraction: 80 × 19
[39m
##
[90m#
[90mFeatures=220 | Cells=80 | Active assay=SCT | Assays=RNA, SCT
[0m
[39m
## .cell orig.ident nCount_RNA nFeature_RNA RNA_snn_res.0.8 letter.idents groups
##
[3m
[90m<chr>
[39m
[23m
[3m
[90m<fct>
[39m
[23m
[3m
[90m<dbl>
[39m
[23m
[3m
[90m<int>
[39m
[23m
[3m
[90m<fct>
[39m
[23m
[3m
[90m<fct>
[39m
[23m
[3m
[90m<chr>
[39m
[23m
##
[90m 1
[39m ATGC… SeuratPro… 70 47 0 A g2
##
[90m 2
[39m CATG… SeuratPro… 85 52 0 A g1
##
[90m 3
[39m GAAC… SeuratPro… 87 50 1 B g2
##
[90m 4
[39m TGAC… SeuratPro… 127 56 0 A g2
##
[90m 5
[39m AGTC… SeuratPro… 173 53 0 A g2
##
[90m 6
[39m TCTG… SeuratPro… 70 48 0 A g1
##
[90m 7
[39m TGGT… SeuratPro… 64 36 0 A g1
##
[90m 8
[39m GCAG… SeuratPro… 72 45 0 A g1
##
[90m 9
[39m GATA… SeuratPro… 52 36 0 A g1
##
[90m10
[39m AATG… SeuratPro… 100 41 0 A g1
##
[90m# ℹ 70 more rows
[39m
##
[90m# ℹ 12 more variables: RNA_snn_res.1 <fct>, nCount_SCT <dbl>,
[39m
##
[90m# nFeature_SCT <int>, SCT_snn_res.0.8 <fct>, seurat_clusters <fct>,
[39m
##
[90m# PC_1 <dbl>, PC_2 <dbl>, PC_3 <dbl>, PC_4 <dbl>, PC_5 <dbl>, tSNE_1 <dbl>,
[39m
##
[90m# tSNE_2 <dbl>
[39mNow we can interrogate the object as if it was a regular tibble data frame.
##
[90m# A tibble: 6 × 3
[39m
## groups seurat_clusters n
##
[3m
[90m<chr>
[39m
[23m
[3m
[90m<fct>
[39m
[23m
[3m
[90m<int>
[39m
[23m
##
[90m1
[39m g1 0 23
##
[90m2
[39m g1 1 17
##
[90m3
[39m g1 2 4
##
[90m4
[39m g2 0 17
##
[90m5
[39m g2 1 13
##
[90m6
[39m g2 2 6We can identify cluster markers using Seurat.
Reduce dimensions
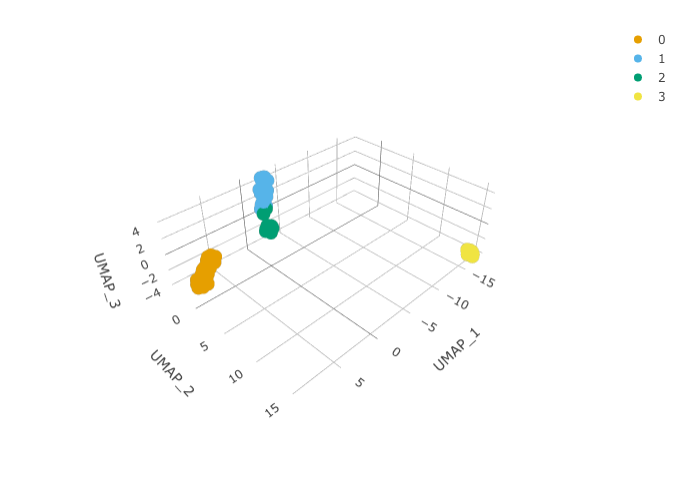
We can calculate the first 3 UMAP dimensions using the Seurat framework.
pbmc_small_UMAP <-
pbmc_small_cluster %>%
RunUMAP(reduction = "pca", dims = 1:15, n.components = 3L)And we can plot them using 3D plot using plotly.
pbmc_small_UMAP %>%
plot_ly(
x = ~`UMAP_1`,
y = ~`UMAP_2`,
z = ~`UMAP_3`,
color = ~seurat_clusters,
colors = friendly_cols[1:4]
)
Cell type prediction
We can infer cell type identities using SingleR [@aran2019reference] and manipulate the output using tidyverse.
# Get cell type reference data
blueprint <- celldex::BlueprintEncodeData()
# Infer cell identities
cell_type_df <-
GetAssayData(pbmc_small_UMAP, slot = 'counts', assay = "SCT") %>%
log1p() %>%
Matrix::Matrix(sparse = TRUE) %>%
SingleR::SingleR(
ref = blueprint,
labels = blueprint$label.main,
method = "single"
) %>%
as.data.frame() %>%
as_tibble(rownames = "cell") %>%
select(cell, first.labels)
# Join UMAP and cell type info
pbmc_small_cell_type <-
pbmc_small_UMAP %>%
left_join(cell_type_df, by = "cell")
# Reorder columns
pbmc_small_cell_type %>%
select(cell, first.labels, everything())We can easily summarise the results. For example, we can see how cell type classification overlaps with cluster classification.
We can easily reshape the data for building information-rich faceted plots.
pbmc_small_cell_type %>%
# Reshape and add classifier column
pivot_longer(
cols = c(seurat_clusters, first.labels),
names_to = "classifier", values_to = "label"
) %>%
# UMAP plots for cell type and cluster
ggplot(aes(UMAP_1, UMAP_2, color = label)) +
geom_point() +
facet_wrap(~classifier) +
my_themeWe can easily plot gene correlation per cell category, adding multi-layer annotations.
pbmc_small_cell_type %>%
# Add some mitochondrial abundance values
mutate(mitochondrial = rnorm(n())) %>%
# Plot correlation
join_features(features = c("CST3", "LYZ"), shape = "wide") %>%
ggplot(aes(CST3 + 1, LYZ + 1, color = groups, size = mitochondrial)) +
geom_point() +
facet_wrap(~first.labels, scales = "free") +
scale_x_log10() +
scale_y_log10() +
my_themeNested analyses
A powerful tool we can use with tidyseurat is nest. We can easily perform independent analyses on subsets of the dataset. First we classify cell types in lymphoid and myeloid; then, nest based on the new classification
pbmc_small_nested <-
pbmc_small_cell_type %>%
filter(first.labels != "Erythrocytes") %>%
mutate(cell_class = if_else(`first.labels` %in% c("Macrophages", "Monocytes"), "myeloid", "lymphoid")) %>%
nest(data = -cell_class)
pbmc_small_nestedNow we can independently for the lymphoid and myeloid subsets (i) find variable features, (ii) reduce dimensions, and (iii) cluster using both tidyverse and Seurat seamlessly.
pbmc_small_nested_reanalysed <-
pbmc_small_nested %>%
mutate(data = map(
data, ~ .x %>%
FindVariableFeatures(verbose = FALSE) %>%
RunPCA(npcs = 10, verbose = FALSE) %>%
FindNeighbors(verbose = FALSE) %>%
FindClusters(method = "igraph", verbose = FALSE) %>%
RunUMAP(reduction = "pca", dims = 1:10, n.components = 3L, verbose = FALSE)
))
pbmc_small_nested_reanalysedNow we can unnest and plot the new classification.
pbmc_small_nested_reanalysed %>%
# Convert to tibble otherwise Seurat drops reduced dimensions when unifying data sets.
mutate(data = map(data, ~ .x %>% as_tibble())) %>%
unnest(data) %>%
# Define unique clusters
unite("cluster", c(cell_class, seurat_clusters), remove = FALSE) %>%
# Plotting
ggplot(aes(UMAP_1, UMAP_2, color = cluster)) +
geom_point() +
facet_wrap(~cell_class) +
my_themeAggregating cells
Sometimes, it is necessary to aggregate the gene-transcript abundance from a group of cells into a single value. For example, when comparing groups of cells across different samples with fixed-effect models.
In tidyseurat, cell aggregation can be achieved using the aggregate_cells function.
pbmc_small %>%
aggregate_cells(groups, assays = "RNA")